JAK 1 and 2 inhibitor superior to adalimumab in treatment of rheumatoid arthritis: The RA-BEAM trial
1. Baricitinib, an oral, once-daily inhibitor of JAK1 and JAK2, has shown promise in the treatment of rheumatoid arthritis (RA).
2. In this randomized controlled trial, baricitinib was superior to injectable adalimumab and placebo, in the amelioration of prespecified RA-related symptoms and radiologic outcomes after 12 weeks of follow-up.
Evidence Rating Level: 1 (Excellent)
Study Rundown: The backbone of RA treatment consists of conventional synthetic disease-modifying antirheumatic drugs (DMARDs), such as methotrexate, and newer biologic DMARDs such as adalimumab, which targets tumor necrosis factor (TNF). RA-BEAM trial is a multicenter randomized controlled trial that compared baricitinib against placebo and adalimumab. Patients with active RA, received background therapy with methotrexate, and were randomized to receive placebo, baricitinib, or adalimumab. The primary outcome was 20% improvement according to the American College of Rheumatology (ACR20) criteria, which occurred significantly more often in the baricitinib group compared to placebo or adalimumab at week 12. There was also significantly reduced radiographic progression of joint damage at week 24 in the baricitinib group compared to placebo. Both baricitinib and adalimumab were associated with a greater number of adverse events, including infection, compared to placebo; baricitinib in particular was associated with neutropenia and lab abnormalities, such as transient increases in creatinine and cholesterol levels.
This drug-company sponsored trial provides promising evidence in support of a once-daily antirheumatic medication in comparison to accepted therapies including an every-other-week anti-TNF therapy and treatment with methotrexate.
Click to read the study, published today in NEJM
Relevant Reading: Safety and efficacy of baricitinib at 24 weeks in patients with rheumatoid arthritis who have had an inadequate response to methotrexate
In-Depth [randomized controlled trial]: This study was conducted over 52 weeks at 281 centers in 26 countries. Participants had RA with an inadequate response to methotrexate patients. A total of 2949 patients were screened for this study and a total of 1305 were treated and qualified for analysis. The ACR20 criteria include a 20% or greater reduction in the number of tender and swollen joints and an improvement of 20% or more in at least three of the following measures: patient assessment of pain, patient global assessment of disease, physical function assessment, and the level of acute-phase reactant.
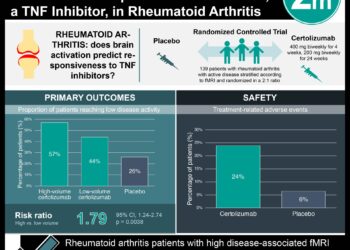
In the primary analysis, at week 12, the ACR20 response rate for baricitinib was 70% compared to 40% for placebo (p < 0.001). Additionally, baricitinib was found to be noninferior to adalimumab (61%) at week 12 for ACR20 response. In further testing with correction for multiple comparisons, baricitinib was superior to adalimumab for ACR20 response (p = 0.01). In secondary analyses, at week 24, both baricitinib and adalimumab achieved significantly reduced radiographic joint damage compared to placebo, with a suggestion of particular benefit in the adalimumab group.
In safety analyses, rates of serious infections were similar between all three groups (1%, 1%, and <1% respectively for placebo, baricitinib, and adalimumab. There were observed reductions in neutrophil counts, and increases in creatinine and liver enzymes (generally transient) in the baricitinib and adalimumab groups compared to placebo. LDL cholesterol levels increased with baricitinib to a greater extent than adalimumab.
Image: CC/Wiki
©2017 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.