Nebulized saline not associated with bronchiolitis hospital length of stay
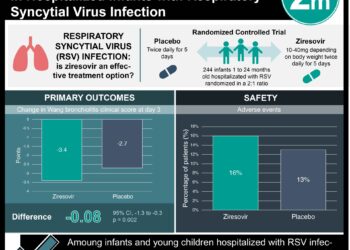
1. No significant difference in length of stay (LOS), adverse events, or readmission was appreciated among hospitalized infants with bronchiolitis who were randomized to receive either hypertonic or normal saline nebulizer treatments.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Bronchiolitis, the most frequent reason for infant hospital admission, is a respiratory condition with no known effective treatment apart from supportive care. Multiple studies have examined the use of interventions such as nebulized 3% hypertonic saline, but findings regarding effectiveness have been inconsistent. Many previous studies have excluded patient with prior wheezing and often investigate saline nebulizer efficacy when used with concomitant bronchodilators. In the current study, researchers aimed to investigate the potential change in LOS associated with hypertonic saline use without concomitant bronchodilators and included those with and without a history of prior wheezing. In comparing individuals who received hypertonic or normal saline nebulized treatments, there were no significant differences in LOS, readmission rates, or adverse events. While results are limited in generalizability as subjects were included from 1 children’s hospital and the study design limited examination of nebulized saline to the acute hospital setting, this study’s findings suggest that, while safe, nebulized hypertonic saline treatments should not be used routinely in the care of infants with bronchiolitis.
Click to read the study, published today in Pediatrics
Relevant Reading: Clinical practice guideline: The diagnosis, management, and prevention of bronchiolitis
In-Depth [randomized, controlled study]: This prospective, double-blinded, randomized controlled study included for analysis data from 190 infants (64% male, mean age = 4.2 months) <12 months of age admitted with bronchiolitis to a tertiary care children’s hospital through the months of November 2011 to June 2014. Individuals treated with steroids, bronchodilators, or those with long-standing cardiopulmonary diagnoses, use of nebulized hypertonic saline <12 hours before presentation, and non-English/non-Spanish speaking families were excluded from the study. Participants were randomized to receive either 4mL of nebulized hypertonic or normal saline every 2 hours as needed with a maximum of 2 treatments per day (n = 93 in the hypertonic saline group, n = 97 in the normal saline group). Primary outcome, LOS, was defined as the time from administration of the first study medication to entry of a discharge order or when the patient met discharge criteria. The investigated secondary outcomes were clinical worsening and readmission. Response to treatment and clinical worsening were assessed using a validated tool, the Respiratory Distress Assessment Instrument (RDAI), and patients were scored both before and 30 minutes after nebulizer treatments. No significant difference in LOS was appreciated between those receiving either nebulized treatment (median LOS was 2.6 days for hypertonic and 2.5 days for normal saline, p = 0.76). There were no significant differences between the treatment groups in readmission rates (n = 4 for hypertonic and n = 3 for normal saline, p = 0.77), adverse event rates (n = 14 for hypertonic vs. n = 12 for normal saline, p = 0.73), and no significant differences in RDAI score changes (0.21 for hypertonic vs. 0.18 for normal saline, p = 0.81).
Image: CC/Wiki/Nevit Dilmen
©2015 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.

![RSV positivity associated with reduced serious bacterial infection risk [Classics Series]](https://www.2minutemedicine.com/wp-content/uploads/2013/09/800px-Respiratory_syncytial_virus_01-350x250.jpg)