One-year of Herceptin preferable to two-year regimen
Image: PD
1. One year of adjuvant Herceptin (trastuzumab) has similar efficacy as a two-year regimen.
2. One-year regimen was associated with a lower incidence of adverse events.
Evidence Rating Level: 1 (Excellent)
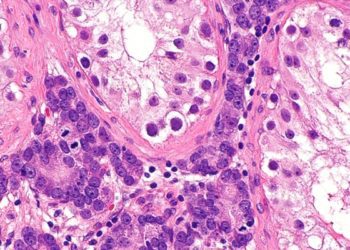
Study Rundown: This large scale, randomized controlled trial found that two years of Herceptin treatment has an unfavorable risk-benefit profile compared with one year of treatment. These results add to the previously published landmark analyses of the HERA trial which demonstrated the efficacy of Herceptin, a monoclonal antibody against the HER2/neu receptor, as an adjuvant treatment for HER2-positive breast cancer.
This study is the first to evaluate the potential risks and benefits of one versus two years of Herceptin therapy. Strengths include its parsimonious randomized design and long-follow up period. It is limited, however, in that findings from this highly controlled study environment may not hold when applied to real life scenarios. Further research might focus on the efficacy of Herceptin in combination with other treatments.
Click to read the study in The Lancet
Click to read an accompanying editorial in The Lancet
Click to read about this trial on Clinicaltrials.gov
Relevant Reading: Up-to-date: Adjuvant medical therapy for HER2-positive breast cancer
Lead study statistician, Dr. Richard D. Gelber, PhD, talks to 2 Minute Medicine: Harvard Medical School, Professor, Department of Biostatistics and Computational Biology, Dana-Farber Cancer Institute, Boston, MA
“The current report updates [previous HERA trial] results and demonstrates long-term improvement in both disease-free and overall survival with 1-year of adjuvant trastuzumab compared with observation, now at 8 years median follow-up, and shows that 2 years of therapy offers similar efficacy and is associated with more adverse events compared with the 1-year treatment. 1-year of adjuvant trastuzumab therapy should remain the de facto standard duration. Ongoing trials are investigating whether durations shorter than 1 year might be as effective as an entire year, and results of these trials are awaited.”
In-Depth [randomized phase 3 clinical trial]: This international, open-label randomized trial compared two years of adjuvant Herceptin (trastuzumab) with the standard one year of adjuvant treatment for HER2-positive breast cancer. A total of 5,102 women were assigned to none (n=1,744), one year (n=1,552), or two years (n=1,553) of treatment. Women were randomized on the basis of age, worldwide region, nodal status, chemotherapy type, hormone-receptor status and intention to use endocrine therapy. Primary outcome was disease-free survival after one year; others included overall survival and adverse events.
Disease-free survival events were comparable in the one-year compared with the two-year treatment group (23.6% for both; HR:0.99, CI:0.85-1.14, p=0.86). Grade 3-4 adverse events were more incident in the two-year compared to the one-year treatment group (20.4% vs 16.3%), as were decreases in left ventricular ejection fraction (7.2% vs 4.1%). When comparing no treatment to one-year of treatment, the one-year group had significantly fewer disease-free-survival events (HR=.76, p<0.0001) and overall deaths (HR=0.76, p=0.0005), with a median follow-up of 8 years.
By Maren Shapiro and Leah Hawkins, MD, MPH
© 2013 2minutemedicine.com. All rights reserved. No works may be reproduced without expressed written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT.