#VisualAbstract: Shortened duration of adjuvant chemotherapy in individuals with stage III colon cancer may be associated with noninferior overall survival
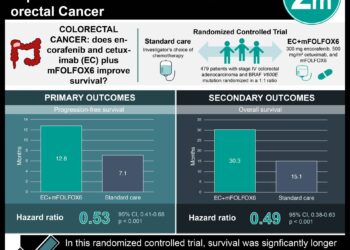
1. Explicit emulation of a target trial investigating the comparative efficacy of a shortened duration of adjuvant chemotherapy in individuals with stage III colon cancer was found to be noninferior to the standard 6-month regimen, consistent with results from the International Duration Evaluation of Adjuvant (IDEA) trial.
2. In contrast, naïve observational analysis of the same comparative efficacy data suggested that a shortened duration of adjuvant chemotherapy was associated with worse overall survival, contraindicating the findings from the IDEA trial.
Evidence Rating Level: 2 (Good)
Study Rundown: For more than a decade, the standard of care for the management of stage III colon cancer has been resection surgery followed by 6 months of adjuvant 5-flourouracil/leucovorin plus oxaliplatin (FOLFOX) or capecitabine plus oxaliplatin (CAPOX) chemotherapy. However, in 2018, clinical practice guidelines were modified as a result of findings from the IDEA trial that analyzed pooled data from 6 randomized clinical trials (RCT) examining the noninferiority of 3 months of adjuvant chemotherapy when compared to the 6 months standard of care. The results and conclusions from the IDEA trial were highly controversial within the oncology community as several real-world observational studies produced findings that contradicted those from the included RCTs. This comparative effectiveness study looked at whether these disparities in overall survival between observational studies and RCTs were attributable to methodological design. From an initial cohort of 3086 patients screened for histologically confirmed stage III colon cancer, 485 patients (16%) were included in the trial. 281 patients (58%) had data consistent with the standard 6-month regimen of adjuvant chemotherapy and 78 patients (16%) had data consistent with the shortened 3-5 months of treatment. The study obtained per-protocol estimates from explicit target trial emulation that were consistent with the intention-to-treat estimates from the IDEA trial for overall survival. In comparison, naïve observational analysis of the same data produced effect size estimates that were meaningfully different from those of the IDEA trial. This highlights the importance of explicitly emulating a target trial when conducting comparative effectiveness research using observational analysis. A major limitation of this study was the relatively small sample size used for trial emulation when compared to the IDEA trial (485 participants vs. 12,834 participants), leading to the possible lack of statistical precision during analysis.
Click to read the study in JAMA
Click to read an accompanying editorial in JAMA
Relevant Reading: Duration of adjuvant chemotherapy for stage III colon cancer
In-Depth [prospective cohort]: This multicenter, prospective comparative efficacy study took place across multiple treatment facilities in the province of Alberta, Canada. From an initial cohort of 3086 patients with histologically confirmed stage III colon cancer diagnosed in Alberta between 2004 and 2015, 485 patients (16%) were included in the trial emulation after screening with inclusion and exclusion criteria similar to that used in the IDEA trial. The median age was 59 years (range, 19-81 years), and 230 (47%) were women. There were 90 deaths in the cohort within a maximum follow-up period of 11.6 years and median overall survival was not reached. The 5-year Kaplan Meier overall survival estimate was 0.79 (95% CI, 0.75-0.84). The primary outcome of this study was to compare the overall survival assessed via vital statistics between a shortened duration of adjuvant FOLFOX or CAPOX chemotherapy ranging from 3 to 5 months compared to the standard 6-month regimen. Results from the trial emulation were then compared with those from a naïve observational analysis that did not explicitly attempt to emulate a hypothetical target trial. In general, the study obtained per-protocol findings from target trial emulation that were consistent with the intention-to-treat findings from the IDEA trial. For example, a shortened duration of adjuvant CAPOX chemotherapy was determined to be noninferior to the current treatment standard of 6 months for overall survival in the IDEA trial (HR, 0.96; 95%CI, 0.85-1.08). Similar findings were produced via trial emulation in this study (HR, 0.96; 95%CI, 0.43-2.14). In contrast, the naïve observational analysis in this study suggested that a shortened duration of adjuvant CAPOX chemotherapy was associated with decreased overall survival for patients (HR, 3.33; 95%CI, 1.04-10.65).
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.