[CORAL-1 trial] Interferon-free regimen for recurrent hepatitis C after liver transplant safe and effective
1. 33 out of 34 post-liver transplant hepatitis C virus (HCV) patients treated with the trial regimen (ombitasavir, ABT-450/r, dasabuvir, ribavirin) had a sustained virology response at 12 and 24 weeks after the end of treatment.
Evidence Rating Level: 1 (Excellent)
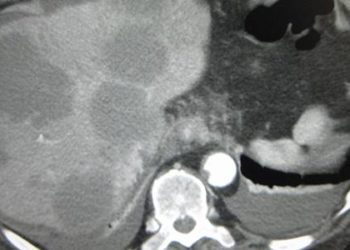
Study Rundown: HCV-related liver failure is a major indication for liver transplants. However, HCV recurrence after transplant is almost guaranteed and is a major cause of graft failure. Treatment regimens for post-transplant HCV recurrence exist, but are limited by side effects and relatively low response rates.
Recent phase 3 trials (SAPPHIRE I and II, PEARL III and IV, TURQOISE II) have demonstrated the effectiveness of a regimen of ombitasavir, ABT-450/r, dasabuvir and ribavirin (AOD+RBV) for HCV genotype I in a variety of clinical settings. This trial investigated the use of AOD+RBV for treatment of HCV recurrence in the post-transplant population.
AOD+RBV was found to be highly effective and safe, with 33 out of 34 patients having undetectable viral load 12 weeks after the end of treatment. Only one patient discontinued the treatment due to side effects. It should be noted that the treatment regimen does require active management of the dosing of ribavirin and of the patients’ immunosuppressive drugs. Monitoring and management may not be as stringent outside of the trial setting.
Click to read the study, published today in NEJM
Relevant Reading: Therapy of Hepatitis C — Back to the Future
In-Depth [ randomized controlled trial]: This study enrolled 34 patients from centers in the US and Spain with HCV genotype 1 infection and who had received a liver transplant in the previous 12 months for chronic HCV infection. Exclusion criteria included co-infection with HIV or hepatitis B virus or multi-organ transplantation. The patients were predominantly white males.
The treatment regimen consisted of: ombitasavir, a NS5A inhibitor; dasabuvir, a nonnucleoside NS5B polymerase inhibitor; ABT-450/r, a protease inhibitor boosted with ritonavir to prolong its availability; and ritonavir. Initial dosing of ribavirin and subsequent changes were determined by investigators for each patient. 19 patients had their doses of ribavirin changed; 9 had their doses changed for anemia (a common side effect of ribavirin). 5 patients required erythropoietin due to low hemoglobin levels.
More from this author: Early risk factor for progression of cystic fibrosis identified, Gut microbes implicated in stroke and heart attacks: new dietary link, New leukemia mutation offers therapeutic targets, Childhood ADHD associated with increased risk of suicide, A marker of aggressive liver cancer and potential therapeutic target identified
Image: PD
©2014 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. No article should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2 Minute Medicine, Inc.