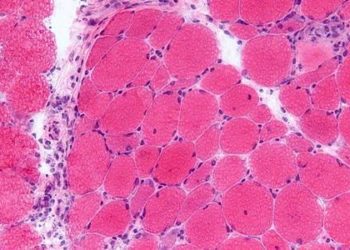
Lirentelimab may be effective for treatment of eosinophilic gastrointestinal disease
1. Patients who received AK002, lirentelimab, had far greater reductions in both gastrointestinal eosinophil burden and gastrointestinal symptoms compared to those who received placebo.
2. These between-group differences emerged immediately following the first dose and persisted across the entire 4-month study.
Evidence Rating Level: 1 (Excellent)
Study Rundown: While eosinophilic esophagitis has become well-characterized over the past decade, recent studies have shown that eosinophilic gastroenteritis may remain underdiagnosed despite causing significant quality-of-life impairments. Diet modification and corticosteroids are commonly used for management of the condition, but no specific pharmacologic therapies have yet been approved. AK002 (lirentelimab) is a first-in-class, humanized, non-fucosylated IgG1 anti–Siglec-8 monoclonal antibody that has been shown to selectively deplete eosinophils and inhibit mast-cell activation, thus reducing degranulation, secretion of inflammatory mediators, and recruitment of other immune cells. In this study, adults with eosinophilic gastritis or duodenitis received high or low dosage AK002 or placebo at monthly intervals. By day 99, two weeks after the fourth and final dose, the combined AK002 group had experienced a significantly greater reduction in gastrointestinal eosinophil count versus placebo. A histologic response was observed in over 90% of patients who received AK002 and only 15% of those who received placebo. Interestingly, patients with concomitant esophageal eosinophilia also had a far greater rate of esophageal histologic remission when treated with AK002, likely owing to a rapid decrease in blood absolute eosinophil count that was not observed in the placebo group. The combined AK002 group also had over twice as much improvement in total gastrointestinal symptom score compared to placebo. Infusion-related reactions were generally mild-to-moderate and occurred far more frequently among those who received AK002, but serious adverse events occurred at similar rates between groups. The primary limitation of this study was the small sample size; further research should be done to confirm the safety and efficacy of this therapeutic agent.
Click here to read the study in NEJM
In-Depth [randomized controlled trial]: In this phase 2 trial conducted at 17 sites in the United States, 65 adults with moderate-to-severe symptoms of eosinophilic gastrointestinal disease were randomly assigned in a 1:1:1 ratio to receive four monthly intravenous infusions of low-dose AK002 (0.3, 1, 1, and 1 mg per kg), high-dose AK002 (0.3, 1, 3, and 3 mg per kg), or placebo. Of the 65, 10 had a diagnosis of gastritis, 25 had a diagnosis of duodenitis, and 30 had concurrent gastritis and duodenitis. Patients with celiac disease, active Helicobacter pylori infection, or inflammatory bowel disease were excluded. The groups had similar clinical characteristics at baseline with the exception of peripheral blood eosinophil count, which was numerically higher in the combined AK002 group. At day 99, the AK002 group had a change in the gastrointestinal eosinophil count of −95%, as compared with 10% in the placebo group (least-squares mean difference, −105 percentage points; 95% CI, −128 to −83; P<0.001). AK002 treatment produced a histologic response (<30 gastrointestinal eosinophils per high-power field) in 37 patients while only 3 patients in the placebo group experienced the same effect. The first secondary endpoint of treatment response (defined as both a >30% reduction in total symptom score and a >75% reduction in gastrointestinal eosinophil count) was observed in 27 patients in the AK002 groups versus only 1 in the placebo group (percentage difference, 64; P<0.001). Lastly, patients in the AK002 groups had greater improvements in all 8 components of the 36-Item Short Form Survey quality-of-life questionnaire compared to those who received the placebo.
Image: PD
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.