Non-toxigenic spores may prevent recurrent Clostridium difficile infection
1. In this study, spores of non-toxigenic Clostridium difficile (C. difficile; M3 strain) administered for prevention of recurrent C. difficile infection were generally well tolerated with adverse events occurring in only 3% of individuals receiving this intervention.
2. Rates of recurrent C. difficile infection were lower in individuals receiving non-toxigenic C. difficile spores than in those receiving placebo in this study.
Evidence Rating Level: 1 (Excellent)
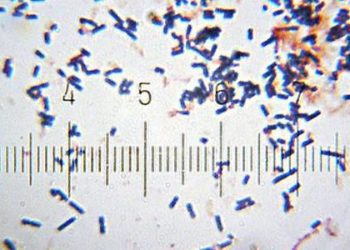
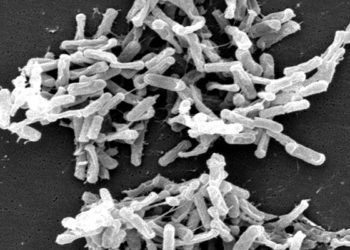
Study Rundown: Clostridium difficile (C. difficile) is an important cause of a potentially fatal healthcare acquired diarrheal illness. There are several therapies available for C. difficile infection, but the morbidity and mortality of C. difficile illness remains high. This randomized, double-blind, placebo-controlled study utilized spores from the non-toxigenic M3 strain of C. difficile (NTCD-M3) in patients with a history of C. difficile infection to prevent recurrence of infection with toxin producing strains of the bacteria.
In this study, rates of recurrent C. difficile infection were lower in individuals receiving non-toxigenic C. difficile spores than in those receiving placebo in this study. Serious adverse events occurred in 3% of NTCD-M3 participants and none of these adverse outcomes were thought to be due to the study intervention. Strengths of this study include the double-blind, randomized, placebo-controlled methodology, use of several doses of NTCD-M3, and careful follow up of clinical adverse events and microbiological data from participants. The major weakness of the study is its relatively small sample size. Ultimately, this study suggests that the administration of NTCD may be safe and effective in preventing recurrent C. difficile infection, but a larger study should be done to further support these findings.
Click to read the study, published today in JAMA
Relevant Reading: Burden of Clostridium difficile infection in the United States.
In-Depth [randomized controlled trial]: This double-blind, placebo-controlled study randomized individuals from 44 centers in the US, Europe, and Canada with a history of C. difficile infection to receive one of three doses of non-toxigenic C. difficile (M3 strain) or placebo. A total of 168 individuals completed the study and were assessed for adverse events as the primary outcome. Serious adverse events occurred in 7% of placebo participants and 3% of NTCD-M3 participants. There was one death in the placebo and NTCD group, and neither death was thought to be associated with the intervention. C. difficile infection recurrence was 30% in the placebo group and 11% in the NTCD-M3 group (OR 0.28; 95%CI 0.11-0.69; p = 0.006). Among recipients of the NTCD-M3 solution, recurrence was 2% for individuals who became colonized with NTCD and 31% (similar to recurrence rate for placebo of 30%) for those who did not (OR 0.01; 95%CI 0.00-0.05; p < 0.001). The recurrence rate was lowest in the group receiving 107 spores/day for 7 days (5%, OR 0.1; 95%CI 0.0-0.6; p = 0.01).
Image: PD
©2015 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.

![[Physician Comment] The extent of C. difficile infections may not differ in light of immune status](https://www.2minutemedicine.com/wp-content/uploads/2012/11/x800px-Clostridium_difficile_01-e1353335581537.jpg.pagespeed.ic_.We5DxZl6Ye-350x250.jpg)
![Radiofrequency catheter ablation effective as first-line therapy for atrial fibrillation [RAAFT-2 trial]](https://www.2minutemedicine.com/wp-content/uploads/2014/02/afib_-75x75.jpg)