On-demand preexposure prophylaxis decreases HIV infection in high-risk men
1. HIV-1 acquisition rates were significantly lower in men who used tenofovir-disoprovil fumarate (TDF) and emtricitabine (FTC) preexposure prophylaxis (PrEP).
2. TDF-FTC was associated with significantly higher rates of gastrointestinal (GI) complaints and elevated serum creatinine levels.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Studies have shown potential for effective PrEP of HIV infections with antivirals such as TDF or FTC. However, these studies examined daily oral regimens, which may underestimate efficacy due to issues with daily adherence. An on-demand program of PrEP may be more efficacious as issues with adherence could be lower, especially in high-risk populations such as men who have sex with men (MSM). This double-blind randomized controlled trial examined the efficacy and safety of sexual activity-dependent PrEP with TDF-FTC in MSM.
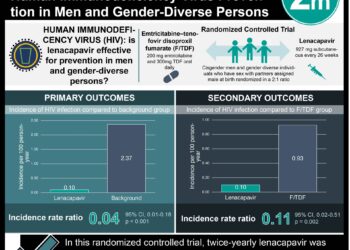
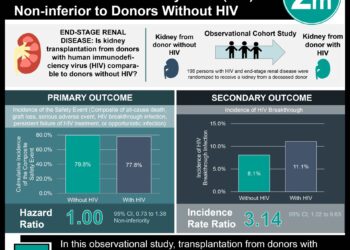
The acquisition rates of HIV-1 were significantly decreased in men who used TDF-FTC before and after sexual activity as compared to placebo: a relative risk reduction of 86%. There were no significant differences in serious adverse events and no deaths in the study. The TDF-FTC group had higher rates of GI adverse events (nausea, vomiting, diarrhea, abdominal pain) and renal adverse events (elevations in serum creatinine). Strengths of this study include examining a novel manner of drug administration in a HIV high-risk group. Limitations include a relatively short follow-up, which does not provide information on long-term adherence.
Click to read the study, published today in NEJM
Relevant Reading: Changes in glomerular kidney function among HIV-1 uninfected men and women receiving emtricitabine-tenofovir disoproxil fumarate preexposure prophylaxis
In-Depth [randomized controlled trial]: This double-blind, modified intention-to-treat, randomized controlled trial was conducted at six study sites in France and one in Canada from February 2012 to October 2014. Inclusion criteria were HIV-negative status men or transgendered female sex adults who have sex with men. Those with hepatitis B or C infection, chronic kidney disease or specific transaminase abnormalities were excluded. Men were randomized to either TDF-FTC or placebo. All participants received risk-reduction counselling and condoms and regularly tested for HIV and other sexually transmitted infections. The primary endpoint was the diagnosis of HIV-1 infection defined as the first evidence of HIV antibodies or p24 antigen in serum as determined by an ELISA assay.
A total of 400 participants who did not have HIV infection were enrolled: 199 in the TDF-FTC and 201 in the placebo group. The median follow-up was 9.3 months (interquartile range [IQR] 4.9 to 20.6). Participants in both the TDF-FTC and placebo group took a median of 15 pills per month (p=0.57). Of the 16 HIV-1 infections that occurred during follow-up, 2 occurred in the TDF-FTC group (incidence 0.91 per 100 person-years) and 14 in the placebo group (incidence 6.60 per 100 person-years), a relative reduction of 86% (95% [CI] 40-98%, p=0.002). There were no significant differences between groups in the frequency of serious adverse events, including zero deaths in the study period. The TDF-FTC group did have significantly higher rates of gastrointestinal events (14% vs. 5%, p=0.002) and significant increases in serum creatinine (18% vs. 10%, p=0.03).
Image: PD
©2015 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.