Phase 3 trial fails to demonstrate benefit of semagacestat in Alzheimer’s disease
Image: PD
1. Use of semagacestat was associated with dose-relevant clinical worsening.
2. There were also renal and immunologic effects with semagacestat use, many of which persisted long after termination of treatment.
Evidence Rating Level: 1 (Excellent)
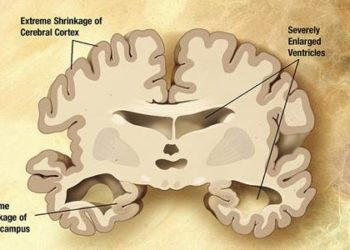
Study Rundown: In this phase-3 clinical trial, semagacestat did not improve cognitive or functional outcomes in patients with Alzheimer’s disease. Additionally, semagacestat was associated with an increased risk of skin cancer and infection, among other adverse events. Fortunately, an earlier-than-planned futility analysis ended the trial early and prevented unnecessary harm. The early termination resulted in completion rates of less than 40%. This limits the power of statistical analyses as some of the primary outcomes had P>0.05. The study also did not find out the mechanisms for such clinical worsening.
Click to read the study, published today in NEJM
Relevant Reading: Phase 2 safety trial targeting amyloid beta production with a gamma-secretase inhibitor in Alzheimer disease
In-Depth [phase 3 randomized study]: This study assessed the safety and efficacy of semagacestat in treating Alzheimer’s disease. 1537 patients underwent randomization to receive semagacestat 100 mg qd, 140 mg qd, or placebo over 76 weeks.
The cognitive ADAS-cog scores worsened in all three groups. The mean changes were 6.4 for placebo, 7.5 for semagacestat 100 mg qd, and 7.8 for 140 mg qd (P=0.15 for 100 mg vs. placebo and 0.07 for 140 mg vs. placebo). The functional ADCS-ADL scores also worsened in all three groups. The mean changes were -9.0 for placebo, -10.5 for 100 mg qd, and -12.6 for 140 mg qd (P=0.14 for 100 mg vs. placebo and <0.001 for 140 mg vs. placebo).
Semagacestat was associated with weight loss (P<0.001). ECGs showed a small increase in QTc interval (P<0.001). The semagacestat group had more cancers and infections. There was evidence for Fanconi’s syndrome and hepatocellular injury. There were also immunological alterations with reduction in IgG levels and lymphocyte counts.
The trial ended early after futility analysis indicated that semagacestat could not show benefit.
By Xiaozhou Liu and Marc Succi
More from this author: Nivolumab plus ipilimumab shows promise for metastatic melanoma, Prophylactic platelet transfusions prevent bleeding in hematologic cancers, Azithromycin is not associated with increased cardiovascular death in low-risk groups, Health education module reduces parasitic infections, New chemotherapy precludes the need for radiotherapy in primary mediastinal B-cell lymphoma
© 2013 2minutemedicine.com. All rights reserved. No works may be reproduced without expressed written consent from 2minutemedicine.com. Disclaimer: We present factual information directly from peer reviewed medical journals. No post should be construed as medical advice and is not intended as such by the authors, editors, staff or by 2minutemedicine.com. PLEASE SEE A HEALTHCARE PROVIDER IN YOUR AREA IF YOU SEEK MEDICAL ADVICE OF ANY SORT.