Pre-exposure prophylaxis (PrEP) effective in preventing HIV infection in high-risk gay men
1. In this randomized open-label trial, pre-exposure prophylaxis (PrEP) with once daily tenofovir-emtricitabine was found effective in preventing HIV-1 infection in high-risk gay men.
2. In the group using PrEP, an increased rate of sexual partners or sexually transmitted disease was not observed.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Although treatment for HIV infected individuals has drastically improved, lifelong care and close clinical observation for these patients is still a necessity. For this reason, primary prevention of this chronic illness would result in a significant reduction in healthcare costs. For men who have sex with men, there is an increased incidence of HIV infection. Previous studies have demonstrated that pre-exposure prophylaxis in this high-risk group can reduce infection rates. This study aimed to retest those findings and examine the “risk compensation” hypothesis. The risk compensation theory hypothesizes that men receiving PrEP will engage in riskier sexual practices because of a perception of increased protection.
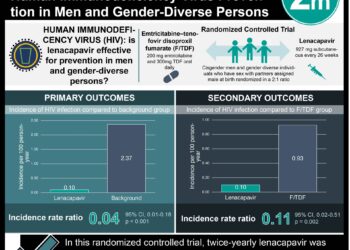
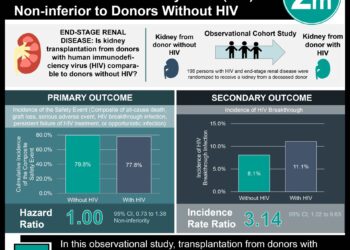
To study this, the investigators completed a randomized open-label trial at 13 sexual health clinics in England. Participants included men who had sex with men over the age of 18 who had anal intercourse without a condom in the past 90 days. Participants were randomly assigned to receive PrEP (tenofovir-emtricitabine) immediately or after a deferral period of one year. The primary outcomes were participant accrual rate and retention. Secondary outcomes including HIV infection during the yearlong deferral period, PrEP adherence, and risk compensation. The investigators found that PrEP was effective in preventing HIV-1 infection (relative reduction of 86% compared to the deferral group), and based on the effectiveness of the prophylaxis, the trial steering committee recommended offering the drug to participants in the deferral group. The results did not support the “risk compensation” hypothesis. Incidence of sexually transmitted disease was no higher in the group who immediately received prophylaxis.
The study was funded by MRC Clinical Trials Unit at UCL, Public Health England, and Gilead Sciences.
Click to read the study, published today in The Lancet
Relevant Reading: Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men
In-Depth [randomized controlled trial]: This study investigated the use of HIV pre-exposure prophylaxis (PrEP) in a high-risk group of men who have sex with men. The study enrolled 544 participants. Follow-up was completed in 94% of the participants in the group who immediately received PrEP and in 90% of the deferral group.
Baseline characteristics were well-balanced between groups. HIV incidence was significantly lower in the immediate group (1.2 cases per 100 person-years, 90% CI 0.4 – 2.9), compared to the deferred group (9.0 per 100 person-years, 90% CI 6.1 – 12.8, p =0.0001). This corresponds to a relative reduction of 86% (90% CI 64 – 96, p=0.0001; absolute difference 7.8/100 person-years, 90% CI 4·3 – 11·3). There were no significant adverse reactions to PrEP. In addition, there was no significant difference in number of sexual partners between groups (p = 0.57). There was also no difference in the incidence of sexually transmitted disease between groups. There were 6 hepatitis C infections, 3 in each group.
Image: CC/PLOS
©2015 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.