Quick Take: Efficacy and Long-term Peripheral Sensory Neuropathy of 3 vs 6 Months of Oxaliplatin-Based Adjuvant Chemotherapy for Colon Cancer
1. A shortened course of oxaliplatin-based adjuvant chemotherapy for stage III colon cancer was associated with decreased peripheral sensory neuropathy without compromising effectiveness.
Evidence Rating Level: 1 (Excellent)
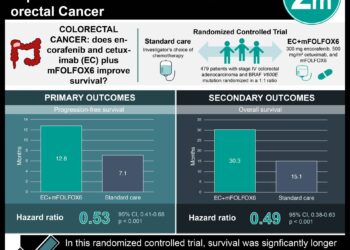
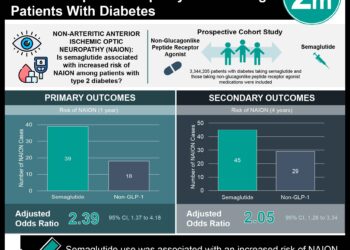
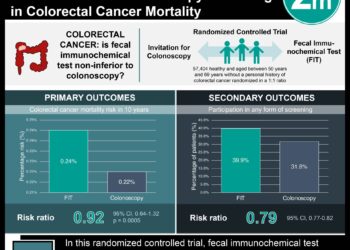
FOLFOX4 regimen, which includes oxaliplatin, has proven efficacy in both the metastatic colorectal cancer setting and the adjuvant setting for resected stage III colon cancers. Peripheral neuropathy is a debilitating associated side effect of oxaliplatin-based chemotherapy. The objective of the ACHIEVE trial was to evaluate the effectiveness and safety of a shortened course of adjuvant oxaliplatin-based chemotherapy in patients with stage III colon cancer. In this open-label multicenter randomized phase III non-inferiority trial based in Japan, 1,313 patients were randomly assigned to receive either 6 months or 3 months of adjuvant chemotherapy with mFOLFOX6 or CAPOX (at physician’s discretion). Treatment regimens were repeated every two weeks for a maximum of 12 or 6 cycles, respectively. The primary efficacy endpoint was disease-free survival, and secondary endpoints included peripheral sensory neuropathy for up to 3 years and overall survival. Of 1,291 treated patients, 969 (75%) of participants received the CAPOX regimen. Researchers found that disease-free survival with 3-month therapy was not significantly different when compared to 6-month therapy (HR 0.95, 95% CI 0.76 to 1.20). The rate of any grade of peripheral neuropathy was 9.7% with 3-month therapy and 24.3% with 6-month therapy (p<0.001), with significantly lower incidences with CAPOX therapy than with mFOLFOX therapy with both durations. The findings of this study therefore indicate that a shorter duration of therapy (3 months) with oxaliplatin-containing chemotherapy may confer significantly lower rates of peripheral sensory neuropathy without compromised effectiveness.
Click to read the study in JAMA Oncology
Image: PD
©2019 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.