#VisualAbstract: Nirmatrelvir does not decrease symptom duration of COVID-19
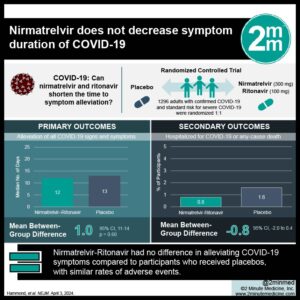
1. In this randomized controlled trial, there was no difference in the median time to sustained alleviation of coronavirus disease 2019 (COVID-19) symptoms between participants who received nirmatrelvir versus placebo.
2. The incidence of adverse events was similar in the nirmatrelvir and placebo groups, with dysgeusia being the most common event associated with nirmatrelvir.
Evidence Rating Level: 1 (Excellent)
Study Rundown: COVID-19, an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), remains a threat to global health, with severe cases contributing to significant morbidity and mortality. Persons of older age and those with underlying conditions such as cardiovascular disease, diabetes, and obesity are at higher risk for severe outcomes. Nirmatrelvir is an oral antiviral medication given with ritonavir, a pharmacokinetic enhancer, which inhibits the main protease of SARS-CoV-2 essential for viral replication. A recent study showed the significant efficacy of nirmatrelvir-ritonavir in reducing rates of COVID-19 progression to hospitalization or death in unvaccinated adults with at least one risk factor for severe COVID-19. This phase II-III trial investigated the efficacy and safety of nirmatrelvir-ritonavir in a new subset population involving individuals who were either at standard risk for severe COVID-19 or those who were fully vaccinated and had at least one risk factor for progression to severe disease. Overall, it demonstrated no significant difference in the median time to sustained alleviation of targeted COVID-19 symptoms between participants who received nirmatrelvir-ritonavir and those who received a placebo. The incidence of adverse events was similar in the nirmatrelvir and placebo groups, with the most common adverse events associated with nirmatrelvir being dysgeusia and diarrhea. The effectiveness of participant blinding in the study may have been limited by the distinct taste of nirmatrelvir-ritonavir, and the generalizability of the results may also be limited by the predominance of the delta variant of SARS-CoV-2 during the study period.
Click to read the study in NEJM
In-Depth [randomized controlled trial]: This randomized controlled trial investigated the safety and efficacy of nirmatrelvir-ritonavir in non-hospitalized adults with symptomatic COVID-19 who were either at standard risk for severe disease or were fully vaccinated and had at least one risk factor for progression to severe disease. Participants were eligible if they were at least 18 years of age, had confirmed SARS-CoV-2 infection, and associated signs or symptoms occurring five or fewer days before randomization. Enrolled patients were randomly assigned in a 1:1 ratio to receive either nirmatrelvir-ritonavir or placebo every 12 hours for five days. The primary endpoint was the median time to sustained alleviation of all targeted COVID-19 signs and symptoms through day 28 based on daily records kept by the participants. Among 1296 participants who underwent randomization, 1288 received at least one nirmatrelvir-ritonavir (654) or placebo (634) and had at least one post-baseline visit. Demographic and baseline participant characteristics were similar in both groups. Overall, the difference in the median time to sustained alleviation of target COVID-19 signs and symptoms through day 28 between the nirmatrelvir-ritonavir group (12 days) and placebo group (13 days) was not significant (p=0.60). Moreover, 5 of 654 participants (0.8%) in the nirmatrelvir-ritonavir group and 10 of 634 participants (1.6%) in the placebo group had COVID-19-related hospitalization or death from any cause (difference, -0.8 percentage points; 95% Confidence Interval, -2.0 to 0.4). The incidence of adverse events during the treatment period was 25.8% in the nirmatrelvir-ritonavir group and 24.1% in the placebo group, with serious adverse events occurring in 1.2% and 1.9% of participants in the respective groups. The most common adverse events in the nirmatrelvir-ritonavir group were dysgeusia, diarrhea, and nausea. In summary, nirmatrelvir did not decrease the duration of signs and symptoms of COVID-19 in adult outpatients.
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.