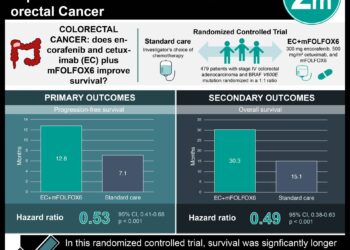
#VisualAbstract: Pembrolizumab linked to longer cancer-free period in advanced MSI-H-dMMR colorectal cancer
1. In a phase 3 trial across 23 countries, Pembrolizumab displayed a longer cancer-free period when compared to the first line therapy for metastatic microsatellite instability or mismatch-repair deficiency (MSI-H-dMMR) colorectal cancer.
Evidence Rating Level: 1 (Excellent)
Study Rundown: A subset of colorectal cancer tumors is mismatch-repair deficiency (dMMR) which has been known to be the leading cause of colorectal cancer in 15% of those diagnosed. However, there is sufficient evidence that suggests that current first line chemotherapy does not produce the desired response. This study was conducted as a multicenter, open-label, randomized control trial that compared pembrolizumab with the recommended first line chemotherapy as suggested by the investigator for MSI-H-dMMR metastatic colorectal cancer. The follow up period for this study was 32.4 months where the patients were randomized into standard-of-care chemotherapy or pembrolizumab. This study also examined adverse effects, disease progression, mean survival, progression-free survival, overall survival, response rate, and efficacy among other things. Overall, pembrolizumab displayed a higher response rate when compared to traditional chemotherapy. Similar adverse events were noted in both groups.
This study was successful at randomizing a large sample size and collecting data over a long course.
Click to read the study in NEJM
In-Depth [randomized controlled trial]: This open label, phase 3 trial was conducted across 23 countries where 307 patients were assigned randomly into one of two groups at 192 sites between February 2016 to February 2018. Patients were eligible if over 18 years of age, with radiologically confirmed and diagnosed MSI-H-dMMR stage IV colorectal cancer, with normal to adequate organ function. Patients were excluded from the study if they received previous adjuvant chemotherapy for this condition in the last six months. One group was given 200 mg of pembrolizumab intravenously every 3 weeks. The other group was given chemotherapy (mFOLFOX6, mFOLFOX6 plus bevacizumab, mFOLFOX6 plus cetuximab, FOLFIRI, FOLFIRI plus bevacizumab or FOLFIRI plus cetuximab) every 2 weeks based on the investigator’s choice of first line therapy for metastatic MSI-H-dMMR colorectal cancer. A maximum number of treatments was assigned to Group A, unless the patient wanted to withdraw from trial, or physician’s opinion was to withdraw the patient from the trial, or the patient developed serious side effects or illness during the course of treatment. Further assessments were conducted by using polymerase-chain-reaction-based analysis and imaging along with ongoing evaluation of side effects and/or adverse events. Overall, the power of the study was approximated at 98%, mean age of patients was 63, median time of treatment exposure was 11.1 months in pembrolizumab group and 5.7 months in the chemotherapy group. The study results indicate that the pembrolizumab group displayed longer progression-free survival when compared to the chemotherapy group with an overall response rate of 83% and 35%, respectively. The most common side effects of pembrolizumab were diarrhea, fatigue and nausea versus for the chemotherapy group, diarrhea, nausea and fatigue.
©2020 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.