Weekly semaglutide effective for decreasing body weight in obesity
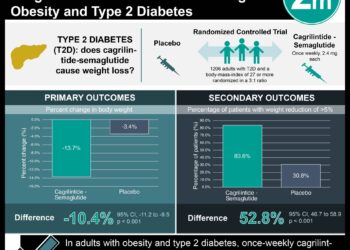
1. Once-weekly treatment of semaglutide was associated with meaningful body weight reduction at 68 weeks compared to placebo in patients with obesity.
2. Semaglutide treatment was associated with more gastrointestinal and hepatobiliary adverse events.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Obesity is increasing in North America and remains significant comorbidity leading to increased metabolic disorders, cardiovascular disease, and malignancies. Semaglutide, a glucagon-like peptide-1 (GLP-1) analog, is approved for type 2 diabetes. This study evaluated the efficacy and safety of semaglutide to reduce body weight in patients with obesity and without diabetes. The study determined a clinically meaningful change in the baseline weight compared to placebo. Furthermore, there was greater cardiovascular risk factor reduction in the group treated with semaglutide. Patient physical function scores were also better with semaglutide treatment. Gastrointestinal side effects such as nausea and diarrhea were more common in the treatment group. The study was limited by males being underrepresented, which limited the generalizability of the study. Nonetheless, the study’s results are significant, showing semaglutide and lifestyle intervention compared to lifestyle alone led to a greater reduction in body weight.
Click here to read the study in the NEJM
Relevant Reading: The effect of semaglutide 2.4 mg once weekly on energy intake, appetite, control of eating, and gastric emptying in adults with obesity
In-Depth [randomized controlled trial]: This was a multicenter, randomized, double-blind placebo-controlled trial of 1,961 patients. Patients with a body mass index (BMI) ≥ 30 or BMI ≥ 27 with one of the following weight-related comorbidities: hypertension, dyslipidemia, obstructive sleep apnea, or cardiovascular disease were included in the study. Patients with known diabetes or a glycated hemoglobin level of 6.5% or greater, previous obesity surgical treatment, or use of antiobesity medication within 90 days of enrollment were excluded from the study. Patients were randomized in a 2:1 ratio of either semaglutide and lifestyle intervention or placebo and lifestyle intervention, respectively. The primary endpoints were percentage change in body weight from baseline to week 68 and reduction in body weight of five percent or more in the same time period. The mean change in body weight was -14.9% in the semaglutide group compared to -2.4% in the control group (95% confidence interval [CI] -13.4 to -11.5, P < 0.001). The semaglutide group had more patients with at least 5% weight reduction (86.4%) compared to the placebo group (31.5%) (P < 0.001). Gastrointestinal side effects were more common in the semaglutide group (74.2%) compared to the placebo group (47.9%). Finally, 7.0% in the semaglutide group and 3.1% in the control group stopped treatment owing to adverse events. In summary, this trial demonstrates promising data on the use of semaglutide for obesity management.
Image: PD
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.