2 Minute Medicine Rewind September 30, 2024
1. A higher body roundness index (BRI) trajectory is associated with an increased risk of cardiovascular disease (CVD).
Evidence Rating Level: 2 (Good)
The BRI, first proposed in 2013, is an index used to quantify a person’s body shape by using waist circumference and height which provides a more accurate estimation of an individual’s body fat and visceral fat composition than traditional indices such as the body mass index. Investigations into the longitudinal relationship between BRI trajectory and CVD morbidity remain limited, with current studies being limited to specific local populations. This longitudinal population-based study therefore sought to characterize the relationship between BRI trajectories and CVD incidence in the Chinese national population. 9,935 participants (mean age = 58.85 ± 9.09 years) over the age of 45 from the China Health and Retirement Longitudinal Study (CHARLS) database were identified. Follow-ups were conducted every 2 years between 2011 and 2020 to assess for CVD events based on questionnaire responses. Group-based trajectory modelling was used to identify BRI trajectories from which 3 groups were identified (low-stable, moderate-stable and high-stable). Compared to participants in the low-risk group, those in the moderate-stable and high-stable groups had a 61% (HR, 1.61 [95% CI, 1.47–1.76]) higher risk of incident CVD and a 163% higher risk (HR, 2.63 [95% CI, 2.25–3.07]). The trend remained after adjusting for demographic factors, medical history, medication history and clinical characteristic variables. Overall, this study found that moderate-stable and high-stable BRI trajectories were associated with a greater risk of developing CVD and supports the use of BRI as a novel tool for the assessment of CVD risk.
Early Addition of Selexipag to Double Therapy for Pulmonary Arterial Hypertension
1. The addition of selexipag early to double oral therapy (DOT) with an endothelin receptor antagonist (ERA) plus a phosphodiesterase type 5 inhibitor (PDE5i) was associated with a reduced risk of hospitalization and disease progression in patients with pulmonary arterial hypertension (PAH).
Evidence Rating Level: 2 (Good)
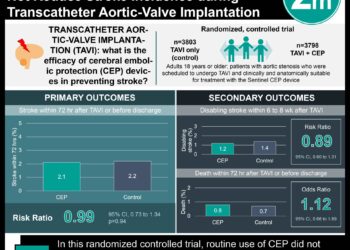
The current standard of care for patients with PAH at low and intermediate risk is DOT with an ERA and a PDE5i. In 2016, oral selexipag was introduced to be added as triple oral therapy (TOT) in patients whose risk of death increases while on DOT. Studies comparing the effectiveness of DOT versus TOT in patients with PAH have been restricted by limited patient populations and confounding variables. This comparative effectiveness study therefore sought to compare the efficacy of TOT versus DOT in patients with PAH in a manner that allows for the delayed addition of oral selexipag. This study attempted to emulate a hypothetical trial in which patients with stable PAH on DOT for 60 days could be randomized to receive TOT or continuous DOT. Data was obtained via medical and pharmacy-dispensed claims between 2015 and 2022 from the Komodo Health payer-complete dataset derived from various private insurers in the United States. 2966 patients (mean [SD] age, 54.3 [14.0] years) were identified and included in the study. Based on unadjusted per-protocol analysis, 14.7% (435 of 2966) of patients in the TOT group experienced hospitalization prior to censoring compared to 25.4% (742 of 2921) in the DOT group. The adjusted hazard ratio (aHR) for PAH-related hospitalization in patients receiving TOT within 6 months versus DOT was 0.81 (95% CI, 0.70-0.95), while the HR for disease progression was 0.82 (95% CI, 0.70-0.95). Overall, this study found that addition of selexipag within 6 months of ongoing DOT for patients with PAH was associated with decreased hospitalization and disease progression.
A Digital Cognitive Behavioral Therapy Program for Adults With Alcohol Use Disorder
1. Over 8 weeks, compared to participants receiving standard outpatient care and weekly clinician-derived cognitive behavioural therapy (CBT), participants receiving web-based CBT did not experience a significant difference in the percentage of days abstinent (PDA) from alcohol per month.
2. Over an 8-month study period, participants assigned to digital CBT showed a greater increase in alcohol abstinence compared to standard outpatient care and clinician-derived CBT.
Evidence Rating Level: 1 (Excellent)
The implementation of CBT into community practice settings for individuals suffering from alcohol use disorder (AUD) has been limited due to time and financial constraints. Digital forms of CBT present alternatives which may improve accessibility to this intervention for patients with AUD. This randomized clinical trial therefore sought to investigate the efficacy of a digital CBT program compared to standard outpatient treatment and clinician-derived CBT. 99 participants with AUD (mean age [SD] = 45.5 [12.7]) were randomized to receive either treatment as usual (TAU), clinician-derived CBT or digital CBT with brief clinical monitoring. Frequency of alcohol use was measured weekly throughout the 8 weeks of treatment and at 1, 3 and 6 month follow-ups. The primary outcome was the PDA by month during treatment and follow-up. The mean (SD) rates of PDA by month from baseline to week 8 were 49.3% (27.8%) to 69.3% (26.2%) for the TAU group, 53.7% (29.8%) to 68.1% (29.9%) for the clinician-derived CBT group and 47.6% (31.8%) to 75.1% (25.1%) for the digital CBT group. At the end of the 8-month study period, digital CBT increased PDA at a faster rate than TAU (b = 2.21; t477 = 2.98; P = .003) and CBT (b = 4.12; t477 = 5.41; P < .001) among those who completed treatment. Overall, this study showed that while there was no significant difference in PDA between treatment groups, digital CBT increased PDA at a faster rate than TAU and clinician-derived CBT, supporting the efficacy of this intervention strategy.
1. Use of the PROVIDE model, an integrated mental health video consultation approach, led to improvements in severity of depression and anxiety symptoms compared to traditional care.
Evidence Rating Level: 1 (Excellent)
Depression and anxiety disorders are among the top contributors to the global burden of disability, yet access to care for such disorders remains limited due to logistical or financial constraints. In recent years, treatment modalities using live interactive videoconferencing have been proposed to mitigate these barriers to access. This multicentre, randomized controlled trial therefore sought to investigate the effectiveness of a novel scalable, integrated mental health video consultation model (PROVIDE) in treating individuals with depression or anxiety. 376 patients were included in the study and randomized to the intervention group receiving PROVIDE or the control group receiving usual care provided by their general practitioner. Patients were deemed eligible if they had at minimum moderately severe depression as defined by the patient health questionnaire-9 (PHQ-9) or moderately severe anxiety as defined by the generalized anxiety disorder (GAD) scale. The primary outcome of the study was the absolute change in the mean severity of depressive and anxiety symptoms from baseline to 6 months after baseline as measured by the patient health questionnaire anxiety and depression scale (PHQ-ADS). At 6 months, the mean change in PHQ-ADS score was -9.2 (95% CI, −10.7 to −7.7) for the intervention group and −7.1 (95% CI, −8.8 to −5.4) for the control group. There was a significant difference in mean change in PHQ-ADS score for the intervention group compared to the control group (adjusted mean change difference = −2.4 points [95% CI, −4.5 to −0.4], P=0.02). Overall, this study found that the use of PROVIDE, a novel integrated mental health video consultation model, for the treatment of individuals with depression or anxiety was associated with improvements in depression and anxiety symptoms compared to the usual care.
1. Treatment of desmoid tumours with dual anti-CTLA-4 and anti-PD-1 blockade with ipilimumab and nivolumab resulted in an overall response rate (ORR) of 18.8% and a clinical benefit rate (CBR) of 62.5%.
Evidence Rating Level: 1 (Excellent)
Desmoid tumours are an extremely rare type of neoplasm originating from fibroblasts. The current standard of care involve active surveillance with surgery being reserved for specific cases. Systemic therapy involving various agents including chemotherapy and tyrosine kinase inhibitors is indicated for cases of unresectable disease. This phase II trial therefore sought to investigate the efficacy of dual anti-CTLA-4 and anti-PD-1 immune checkpoint inhibition on desmoid tumours. 16 patients (median age [range] = 37 [20-82] years) from various centres in the United States were included in the study on the basis of having a histologically confirmed desmoid tumour with no other available treatment options that could improve overall survival (OS) or contraindications to such treatment options. The ORR was 18.8% (3/16) with an overall CBR of 62.5% (10/16). Three patients had a partial response (PR) with seven patients showing evidence of tumour shrinkage. The median progression-free survival (PFS) was 19.4 months (95% CI 8.3 months-not reached). Overall, treatment with ipilimumab and nivolumab in patients with desmoid tumours resulted in an ORR of 18.8% and a CBR of 62.5%, representing a possible additional treatment option for individuals living with desmoid tumours.
Image: PD
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.