Adaptive interim PET-CT guides treatment for advanced Hodgkin’s lymphoma
1. The use of interim PET-CT scanning can de-escalate chemotherapy use during treatment for advanced Hodgkin’s disease by using doxorubicin, vinblastine, and dacarbazine (AVD) instead of doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) after negative scan.
2. Results were slightly below the non-inferiority margin for progression-free survival at 3 years when comparing the omission of bleomycin from ABVD to ABVD alone, but there was decreased pulmonary toxicity and fatigue with AVD.
Evidence Rating Level: 1 (Excellent)
Study Rundown: The typical treatment for advanced Hodgkin’s lymphoma consists of ABVD. While this regimen has a lower cure rate compared to escalated therapy with bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbaczine and prednisone (BEACOPP), the toxicity of the latter only permits its use in certain patient populations. Further, ABVD also comes with increased rates of pulmonary side effects and fatigue. In seeking a chemotherapeutic regimen representing a “middle-ground” between ABVD and BEACOPP, retrospective studies have shown PET avid scans with 18-F deoxyglucose (18-FDG) can predict 2-year progression free survival rates.
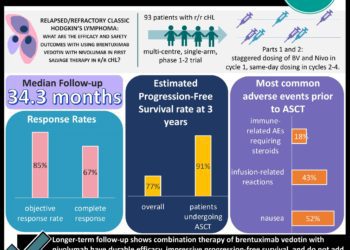
This prospective trial involved patients having advanced Hodgkin’s lymphoma and negative PET-CT scans after 2 cycles of ABVD, where patients were then randomized into either further ABVD or AVD. Those with positive PET-CT scans received BEACOPP. Results demonstrated the AVD regimen fell slightly short of the defined non-inferiority margin compared to ABVD for 3-year progression free survival, but did show decreased pulmonary toxicity and rates of fatigue in the AVD group. Strengths of this trial include randomization, large patient numbers and multiple centers in different countries. The limits of the study reflect the lack of long-term data to fuller ascertain the benefits of AVD. Although the non-inferiority margin fell slightly short, the clinical significance is important as the omission of bleomycin essentially shows no difference in 3-year progression-free survival and decreases pulmonary toxicity and fatigue compared to ABVD. Further, should patients fail AVD, salvage chemotherapy (BEACOPP) is available, thus giving patients multiple options for therapy.
Click to read the study, published today in NEJM
In-Depth [randomized controlled trial]: This study tested interim PET-CT scanning as a means to detect early response to chemotherapy, which would then guide future treatment for those with advanced Hodgkin’s lymphoma. Patients were initially treated with 2 cycles of ABVD chemotherapy. Then, patients with negative PET-CT findings, using a 5-point scale with central image review, were prospectively randomized to either ABVD or AVD chemotherapy. Patients with a positive PET-CT scan were given BEACOPP. The primary outcome was the 3-year progression free survival rate between groups to exclude a non-inferiority difference of 5 of more percentage points.
There were a total of 1214 patients registered in the trial, with 937 of 1119 patients having a negative interim PET-CT scan. The median follow-up was 41.2 months (range 2 to 79.7 months), with 142 events of disease progression, relapse or death occurring; there was no significant difference between the ABVD and AVD groups with respect to these outcomes. The 3-year progression free survival rate in the ABVD group was 85.7% (95%CI 82.1-88.6) and 84.4% (95%CI 80.7 to 87.5) in the AVD group. The absolute difference (ABVD minus AVD) at 3 years was 1.6 percentage points (95%CI -3.2 to 5.3). There was similar 3-year overall survival between the two groups; 97.2% (95%CI 95.1 to 98.4) with ABVD and 97.6% (95%CI 95.6 to 98.7). Respiratory events were more severe in the ABVD group compared to the AVD group. There were a total go 62 patient deaths during the trail, representing a 3-year progression-free survival of 82.6% and overall survival of 95.8%.
Image: PD
©2016 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.