DaxibotulinumtoxinA trial for injection for the treatment of upper limb spasticity in adults after stroke or traumatic brain injury
1. DaxibotulinumtoxinA-lanm 500U showed the greatest reduction in MAS scores in patients suffering from spasticity following stroke and traumatic brain injury
Evidence Rating Level: 1 (Excellent)
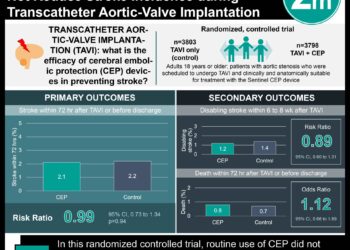
Spasticity is a common outcome of upper motor neuron damage that can happen in stroke, traumatic brain injury, spinal cord injury, and neurodegenerative diseases. It is caused by a hyperexcitability that results in muscle stretch hyperreflexia, velocity-dependent hypertonia, and spastic dystonia. This results in long-term motor function impairment and a reduction in quality of life, activities of daily living, and can increase caregiver burden. The current first-line pharmacotherapy for adults with upper limb spasticity (ULS) is intramuscular botulinum toxin type A injections. This treatment has an effectiveness of 12-13 weeks requiring patients to receive around four injections per year. DaxibotulinumtoxinA-lanm for injection (DAXI) is a new-generation neurotoxin that has been shown to have an extended duration of effect. This double-blind, placebo-controlled, dose-ranging study was used to evaluate three doses of DAXI for adults with ULS post-stroke or TBI. Participants received either a placebo, DAXI 250 U, DAXI 375 U, or DAXI 500 U. Participants, investigators, other clinical staff, and the study sponsor were blinded to treatment assignment. Participants were evaluated at least five times (Weeks 2, 4, 6, 8, and 12) to assess treatment response, tolerability, and safety. Muscle tone was measured using the Modified Ashworth Scale (MAS) in the suprahypertonic muscle (SMG) and the Patient Global Impression of Change (PGIC). All treatment groups showed an improvement from baseline and compared to the placebo at week 6 with DAXI 500U showing the largest reduction. At week 12, the reduction in MAS score from baseline at the primary target SMG was 25.0% (6/24) for placebo, and 40.9% (9/22; p = .25), 36.8% (7/19; p = .43), and 50.0% (9/18; p = .11) for DAXI 250 U, DAXI 375 U, and DAXI 500 U versus placebo, respectively. Mean PGIC scores also significantly improved in all groups from baseline, but no difference was seen between placebo and DAXI dose groups. All DAXI doses were well tolerated. Therefore, DAXI 500U showed the greatest therapeutic effect while maintaining high levels of safety and no adverse effects.
Click to read the study in PMRJ
Image: PD
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.