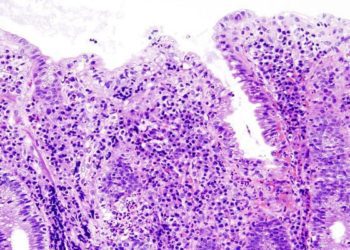
Fecal Microbiota, live-jslm may be an effective means of preventing Clostridiodes difficile infections in patients with inflammatory bowel disease
1. The use of fecal microbiota, live-jslm (RBL; REBYOTA®) in the prevention of recurrent Clostridioides difficile infection (rCDI) in patients with inflammatory bowel disease (IBD) was associated with high efficacy and was well-tolerated.
Evidence Rating Level: 2 (Good)
Study Rundown: Patients with IBD are at an increased risk of developing recurrent rCDI compared to the general population as well as worse clinical outcomes when they do develop CDI. RBL, a fecal microbiota therapeutic modality aimed at restoring the healthy gut microbiome, has previously been shown to be effective in preventing rCDI in adult patients without IBD. This study therefore sought to investigate the effectiveness of RBL in the prevention of rCDI in adult patients living with IBD.
In this open-label, single-arm, prospective study, 697 participants including 74 patients with IBD with documented rCDI received RBL. Outcomes included treatment success and sustained clinical response at 6 months, as well as the number of participants who experienced treatment-emergent adverse events (TEAEs). 78.9% of patients with IBD and 73.2% of patients without IBD experienced treatment succes, with 91.1% of individuals with IBD who achieved treatment success achieving a sustained clinical response at 6 months. Similarly, 91.0% of individuals without IBD who achieved treatment success achieved a sustained clinical response at 6 months. Serious TEAEs were reported in 1.4% of individuals with IBD and 4.2% of individuals without IBD, and were most commonly due to a pre-existing condition.
Overall, this study found that the use of RBL in patients with IBD with confirmed rCDI was effective in preventing rCDI and was well tolerated.
Click to read the study in Inflammatory Bowel Diseases
In-Depth [phase 3, open-label, single-arm clinical trial]: Patients with IBD are at nearly a 5-fold increased risk of developing recurrent rCDI due to a disrupted intestinal microbiome, frequent antibiotic use and other factors. Indeed, when such patients do experience rCDI, patient outcomes are often worse than outcomes in individuals without IBD. RBL is a novel therapeutic strategy involving fecal microbiota transplantation previously shown to be effective in preventing rCDI in adult patients without IBD. This study therefore sought to investigate the effectiveness of RBL in the prevention of rCDI in adult patients living with IBD.
697 participants including 74 patients with IBD (median [IQR] age, 47.0 [38.0-66.0]) and 623 patients (median [IQR] age, 65.0 [51.0-74.0]) without IBD with documented rCDI received RBL. Outcomes included treatment success and sustained clinical response at 6 months, as well as the number of participants who experienced treatment-emergent adverse events (TEAEs). 78.9% (56/71) of patients with IBD and 73.2% (443/605) of patients without IBD experienced treatment succes, with 91.1% (51/56) of individuals with IBD who achieved treatment success achieving a sustained clinical response at 6 months. Similarly, 91.0% (403/443) of individuals without IBD who achieved treatment success achieved a sustained clinical response at 6 months. 45.9% (34/74) of patients with IBD experienced a TEAE, with 23.0% of individuals with IBD experiencing a TEAE due to pre-existing conditions. Likewise, 47.5% (296/623) of patients without IBD experienced a TEAE, with 23.1% of these being due to pre-existing conditions. Serious TEAEs were reported in 1.4% of individuals with IBD and 4.2% of individuals without IBD, and similarly were most commonly due to a pre-existing condition.
Image: PD
©2025 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.