Teplizumab effective in maintaining β-cell function in type 1 diabetes
1. In this randomized-controlled trial, two 12-day courses of teplizumab improved C-peptide levels compared to placebo at week 78 in patients with stage three type 1 diabetes.
2. No significant differences were observed between the teplizumab and placebo groups with respect to secondary clinical outcomes, including clinically relevant hypoglycemic events.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Type 1 diabetes is an autoimmune condition characterized by the T-cell-mediated destruction of pancreatic β-cells with a substantial burden of disease management. Glycemic control tends to be particularly difficult in younger patients, rendering rapid disease progression in these populations. Teplizumab, a humanized anti-CD3 monoclonal antibody, was recently approved by the Food and Drug Administration in patients eight years of age or older with preclinical type 1 diabetes to delay the onset of clinical disease. Previous studies have demonstrated that a short course of teplizumab preserves β-cell function without significant long-term safety effects. This trial investigated whether two short teplizumab courses would preserve β-cell function and improve important clinical outcomes in children with newly diagnosed stage three type 1 diabetes. Overall, it demonstrated that teplizumab improved C-peptide levels compared to placebo at week 78, suggesting preservation of β-cell function. Moreover, no significant differences were observed between the teplizumab and placebo groups with respect to secondary clinical outcomes, including mean insulin dose, change in glycated hemoglobin, percentage of time in the target glucose range, and clinically relevant hypoglycemic events. The generalizability of the study results was limited by a lack of racial diversity among its participants.
Click to read the study in NEJM
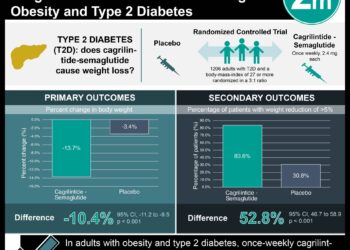
In-Depth [randomized controlled trial]: Provention Bio’s Type 1 Diabetes Trial Evaluating C-Peptide with Teplizumab (PROTECT) is a phase three, randomized, placebo-controlled trial that evaluated the efficacy of teplizumab in the preservation of β-cell function in young patients with newly diagnosed type 1 diabetes. Patients 8 to 17 years of age with stage three disease diagnosed within six weeks were selected. A total of 328 participants were enrolled and randomly assigned to receive teplizumab or placebo in a 2:1 ratio for two 12-day courses, 26 weeks apart. A cumulative dose of 9mg per square meter was administered to each individual. The primary endpoint was defined as the change from baseline in C-peptide levels at week 78, with levels measured from the area under the concentration-time curve (AUC) during a four-hour mixed-meal tolerance test. Secondary clinical endpoints included mean daily insulin dose, change in glycated hemoglobin, percentage of time in the target glucose range, and clinically relevant hypoglycemic events. Treatment with teplizumab resulted in significantly higher C-peptide AUC levels compared to placebo at week 78 (least-squares mean difference, 0.13 pmol per milliliter; 95% Confidence Interval [CI], 0.09 to 0.17; p<0.001). More specifically, 94.9% of patients treated with teplizumab (95% CI, 89.5 to 97.6) exhibited a clinically meaningful peak C-peptide concentration of 0.2 pmol per millimeter or higher compared to 79.2% of patients receiving placebo (95% CI, 67.7 to 87.4). No significant differences were observed for secondary endpoints, including clinically important hypoglycemic events. In summary, the PROTECT study showed that two short courses of teplizumab were effective in preserving β-cell function but not in improving key clinical outcomes.
Image: PD
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.