Topical corticosteroid use in pregnancy not associated with increased risk of small for gestational age or low birth weight newborns
1. No association was found between topical corticosteroid use during pregnancy and increased risks of newborns being small for gestational age (SGA) or having low birth weight, regardless of corticosteroid potency and amount used.
Evidence Rating Level: 2 (Good)
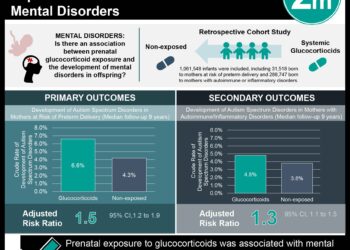
Study Rundown: Topical corticosteroids are frequently used during pregnancy for various dermatological conditions such as eczema. However, data to help evaluate potential fetal harm from topical corticosteroid use in pregnancy are limited and conflicting, in particular with the use of potent to very potent agents. The most recent guidelines recommend the use of topical corticosteroids with mild to moderate potency in pregnancy, whereas potent to very potent agents should be reserved as second-line therapy and used only for a short period. This large cohort study using data from the Danish Medical Birth Registry sought to evaluate whether topical corticosteroid use in pregnancy was associated with increased risks of small for gestational age (SGA) and low birth weight. The primary outcomes were SGA and low birth weight, where associations between outcomes and exposure was assessed via relative risk ratios and absolute risk differences. From a source cohort of 1.1 million live birth singleton pregnancies in Denmark (1997-2016) eligible for study inclusion, 60,497 pregnancies were exposed to topical corticosteroids. This was matched with 241,986 unexposed pregnancies on the basis of propensity scores, including a wide set of baseline characteristics. In the cohort exposed to topical corticosteroids during pregnancy, 5,678 (9.4%) of the delivered infants were born SGA in contrast to 22,634 infants (9.4%) among the matched unexposed pregnancies. Low birth weight occurred in 2,006 (3.3%) of the exposed pregnancies compared to 8,675 (3.6%) of the unexposed pregnancies. Analysis showed that exposure to potent to very potent topical corticosteroids at any amount was not associated with an increased risk of SGA or low birth weight. A limitation of this study and potential source of confounding includes patient cases where the underlying indication being treated, rather than the treatment itself, increases the risk of the outcome (i.e., confounding by indication). Although the study found no significant increased risk in any of the analyses, residual confounding cannot be excluded.
Click to read the study in JAMA Dermatology
Relevant Reading: Pregnancy outcomes after maternal exposure to topical corticosteroids: a UK population-based cohort study
In-Depth [retrospective cohort]: This nationwide cohort study included all pregnancies resulting in live births identified via the Danish Medical Birth Registry from January 1997 to December 2016. From an initial source cohort of 1.1 million live birth singleton pregnancies, a total of 60,497 pregnancies exposed to topical corticosteroids were matched with 241,986 unexposed pregnancies to be included in this study. The median gestational age at first filled prescription for a topical corticosteroid was day 112 (IQR, 44-181 days), with a median received amount of 30 g (IQR, 30-60 g; mean, 53.6 g). A total of 27,630 pregnant women had filled prescriptions for potent to very potent topical corticosteroids (median received amount, 30 g; IQR, 25-80 g; mean, 68.9 g). Among 60,497 pregnancies in the exposed cohort, 5,678 infants (9.4%) were born SGA compared with 22,634 infants (9.4%) in the matched unexposed cohort (RR, 1.00; 95%CI, 0.98-1.03 and ARD, 0.3; 95%CI, -2.3 to 2.9 per 1000 pregnancies). Low birth weight occurred in 2,006 (3.3%) of the exposed pregnancies compared with 8,675 (3.6%) of the unexposed pregnancies (RR, 0.92; 95%CI, 0.88-0.97 and ARD, -2.7; 95%CI, -4.3 to -1.1 per 1000 pregnancies). Furthermore, exposure to potent to very potent topical corticosteroids at any amount was not associated with an increased risk of SGA (RR, 1.03; 95%CI, 0.99-1.07) or low birth weight (RR, 0.94; 95%CI, 0.88-1.00). Lastly, post hoc analyses did not find a significant increased risk among those receiving large amounts of potent to very potent topical corticosteroids throughout pregnancy compared with unexposed pregnancies (RR, 1.17; 95%CI, 0.95-1.46 for SGA and RR 1.14; 95%CI, 0.81-1.60 for low birth weight).
Image: PD
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.