#VisualAbstract: Convalescent plasma is not effective at improving clinical outcomes or reducing mortality of COVID-19 patients not on mechanical ventilation
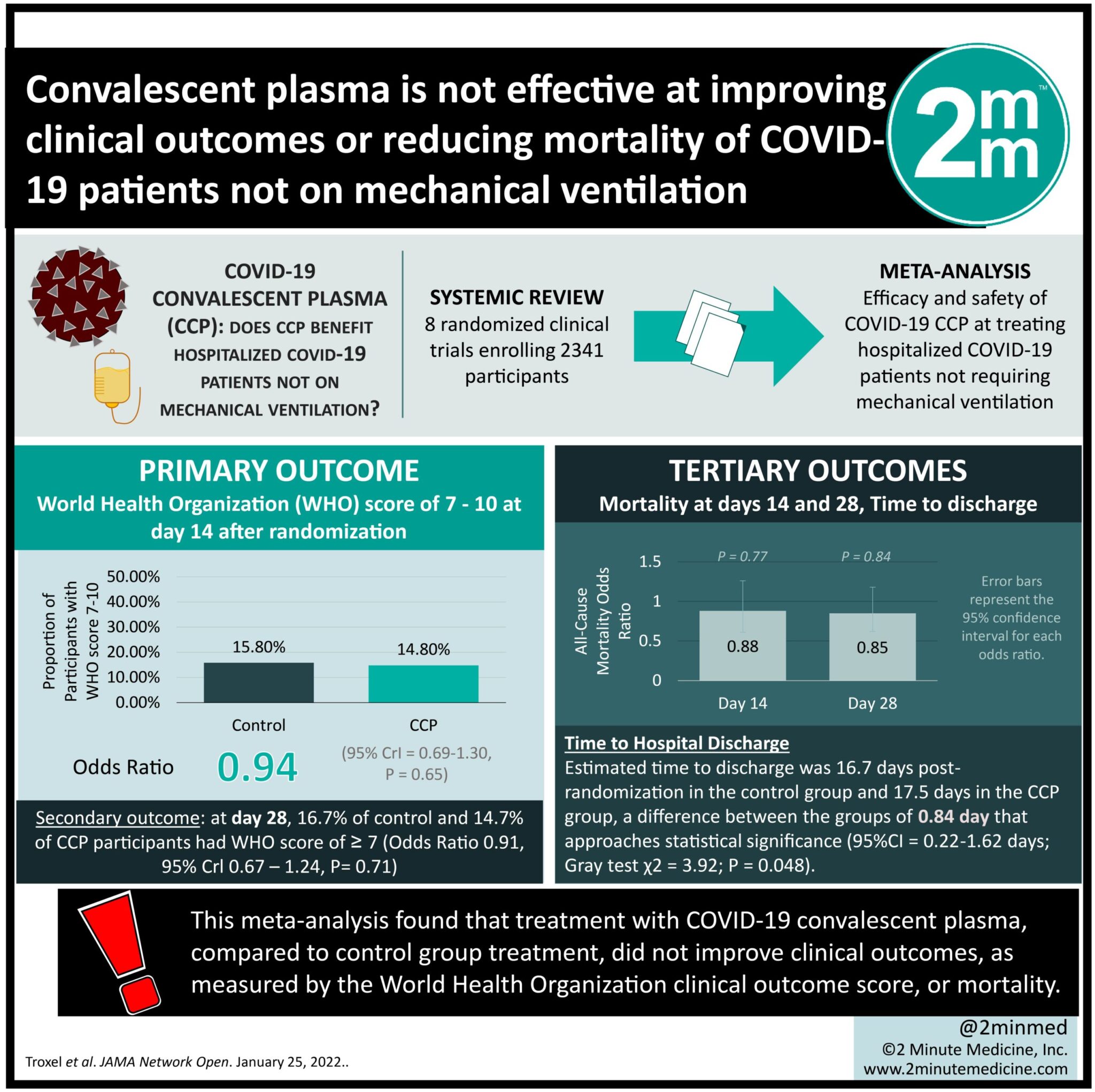
1. This meta-analysis found that treatment with COVID-19 convalescent plasma (CCP), compared to control group treatment, did not improve clinical outcomes, as measured by the World Health Organization clinical outcome score, or mortality.
2. There was an improvement in the mortality and clinical outcomes for participants treated with CCP who had a baseline WHO score of 4 (good clinical level), blood type A, and pre-existing diabetes, cardiovascular, and pulmonary disease, compared to control treatment.
Evidence Rating Level: 1 (Excellent)
Study Rundown: This study, called the COMPILE (COntinuous Monitoring of Pooled International Trials of ConvaLEscent Plasma for COVID-19 Hospitalized Patients) study, pools individual patient data from RCTs in various stages of completeness to expedite information on treating COVID-19. This meta-analysis specifically investigates the efficacy and safety of COVID-19 convalescent plasma (CCP) at treating hospitalized COVID-19 patients not requiring mechanical ventilation. Data were pooled on the 14 and 28-day mortality, time to discharge, and World Health Organization (WHO) 11-point clinical outcome scale (where a higher score represents a worse outcome) of COVID-19 RCT patients treated with CCP or a control treatment. Data were analyzed based on participant progress up to April 2021. Of the 2369 eligible participants from 8 RCTs, 1138 received the control treatment and 1231 received CCP. This meta-analysis found that CCP treatment, compared with control treatment, did not lower the WHO clinical outcome score or mortality. High WHO scores of 7 to 10 occurred in 15.8% of control participants compared to 14.2% of CCP participants on day 14 and 16.7% of control participants compared to 14.7% of CCP participants on day 28; there was no statistically significant difference at either time between the groups. Participant mortality was not significantly different between the groups at both time points, at 8.6% in the control group and 6.7% in the CCP group on day 14 and 13.6% in the control group and 10.9% in the CCP group on day 28. The participants with a baseline WHO score of 4, blood type A, and pre-existing diabetes, cardiovascular, and pulmonary disease benefited from CCP compared to control treatment. One limitation of this study is that COVID-19 mutated during the time of rolling recruitment, which may have decreased the statistical power of this study. The included studies used different methods to measure baseline and post-treatment antibody titres, preventing comparison between studies of this important data. Additionally, since not all included RCTs reported concomitant medications, it is possible that other medications contributed to the measured effects. Finally, the use of discharge WHO score instead of day 14 or 28 WHO score in those lost to follow-up could misclassify outcomes, though the affected sample is small.
Click to read the study in JAMA Network Open
Relevant Reading: COVID-19 convalescent plasma treatment of moderate and severe cases of SARS-CoV-2 infection: A multicenter interventional study
In-Depth [systematic review and meta-analysis]: Patient data from ongoing RCTs on the efficacy of CCP at treating confirmed-COVID patients were pooled on a rolling basis. RCTs were identified from the literature, clinical trial registry sites, and medRxiv. The outcome measures of interest measured at 14 and 28 days included mortality, time to discharge, and the WHO clinical outcomes scale. Covariates in the model analysis included age, sex, baseline WHO score, symptom duration before randomization, and time of enrollment. Of the 8 included RCTs 6 used standard-of-care, 1 used non-convalescent plasma, while 1 used saline solution as the control group. Of the 2369 eligible participants (median age = 60 years, 35.7% women), 19.1% had a baseline WHO score of 4, 63.4% a score of 5, and 17.6% a score of 6. 38 participants were lost to follow-up after discharge from hospital, so their WHO score at discharge was used in place of their day 14 score. This meta-analysis found no association of CCP with better clinical outcomes, represented via lower WHO score. 15.8% of control participants and 14.2% of CCP participants had a WHO score of 7 to 10 on day 14 (WHO score of ≥7: odds ratio [OR] = 0.94, 95%CrI = 0.69-1.30, P = 0.65). The model of WHO score comparing those that received CCP to controls had an odds ratio of 0.94 (95%CrI = 0.74-1.19, P = 0.71). 16.7% of control participants and 14.7% of CCP participants had a WHO score of 7 to 10 on day 28 (WHO score of ≥7: OR = 0.91, 95%CrI = 0.67-1.24, P = 0.74). The model of WHO score by receipt of CCP compared to control had an odds ratio of 0.94 (95%CrI, 0.74-1.19, P = 0.72). The frequency of participant death by day 14 was 8.6% in the control group and 6.7% in the CCP group. By day 28, it remained consistent between the two groups at 13.6% in the control group and 10.9% in the CCP group (stratified log-rank test χ2 = 2.8; P = 0.09). The average day of mortality after discharge was 16.7 in the control group and 17.5 in the CCP group, a difference between the groups of 0.84 day that approaches statistical significance (95%CI = 0.22-1.62 days; Gray test χ2 = 3.92; P = 0.048). All-cause mortality at days 14 and 28 had an odds ratio of 0.88 (95%CrI = 0.61-1.26, P = 0.77) and 0.85 (95%CrI = 0.62-1.18, P = 0.84), respectively. The eligible RCTs had substantial heterogeneity where some subgroups, including those with baseline WHO score of 4, blood type A, and pre-existing diabetes, cardiovascular, and pulmonary disease, benefited from CCP. There was no benefit of CCP in the subgroups stratified by age or by symptom duration. These results were consistent with those in the sensitivity analysis.
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.