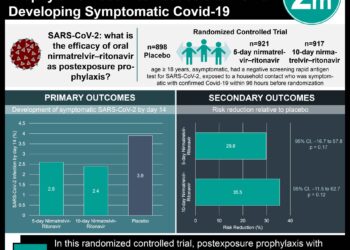
#VisualAbstract: Hospitalized COVID-19 patients show decrease in mortality with dexamethasone
1. Patients hospitalized with COVID-19 and treated with dexamethasone showed an overall decrease in 28-day mortality compared to those treated with usual care.
2. Dexamethasone treatment had a shorter duration in hospital stay compared to the usual care group.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Although the majority of coronavirus disease 2019 (COVID-19) cases are asymptomatic or have minor symptoms, patients need hospital care which can progress to respiratory failure and require ventilatory support. Furthermore, no therapeutic agent has been shown to reduce mortality in this subset of patients. As such, this study assessed the use of dexamethasone in patients hospitalized with COVID-19. The study determined mortality at 28 days was significantly lower in the dexamethasone group compared to the usual care group. Additionally, no benefit was found amongst patients who did not require oxygen. Patients treated with dexamethasone were also noted to have a shorter duration in their hospital stay and a greater probability of being discharged within 28 days. The study was limited by the lack of long-term follow-up in patients treated with dexamethasone. Nonetheless, this study’s results are significant, and its findings have led to updated treatment guidelines for patients hospitalized with COVID-19 on ventilatory support.
Click to read the study in NEJM
Relevant Reading: Scope, quality, and inclusivity of clinical guidelines produced early in the covid-19 pandemic: rapid review
In-Depth [randomized controlled trial]: This controlled, open-label trial enrolled 6,425 patients. Eligible patients included those with confirmed SARS-CoV-2 infection, hospitalized for severe symptoms, and on ventilation or oxygen support. Patients were excluded if dexamethasone was not available at the hospital. Randomization occurred in a 2:1 ratio to receive either usual care alone or usual care plus dexamethasone (oral or IV 6 mg daily), respectively. The primary endpoint was all-cause mortality within 28 days after randomization. Overall, mortality at 28 days was significantly lower in the dexamethasone group (mortality, 482 of 2104 patients; 22.9%) compared to patients in the control group (mortality, 1110 of 4321; 25.7%) (rate ratio, 0.83; 95% confidence interval [Ci], 0.75 to 0.93; P<0.001). Subgroup analysis showed there was no benefit in treating patients who did not receive respiratory support with dexamethasone (17.8%) compared to usual care alone (14.0%) (rate ratio, 1.19; 95% CI, 0.92 to 1.55). Furthermore, patients treated with dexamethasone (median, 12 days) had a shorter duration in the hospital compared to the control group (median, 13 days). Finally, patients treated with dexamethasone had a greater probability of discharge after 28 days compared to usual care alone (rate ratio, 1.10; 95% CI, 1.03 to 1.17). Taken together, dexamethasone was shown to decrease all-cause mortality, shorten the duration of hospital stay, and improve the probability of discharge in hospitalized patients with COVID-19.
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.