#VisualAbstract: The use of COVID-19 convalescent plasma not beneficial to patients hospitalized with COVID-19
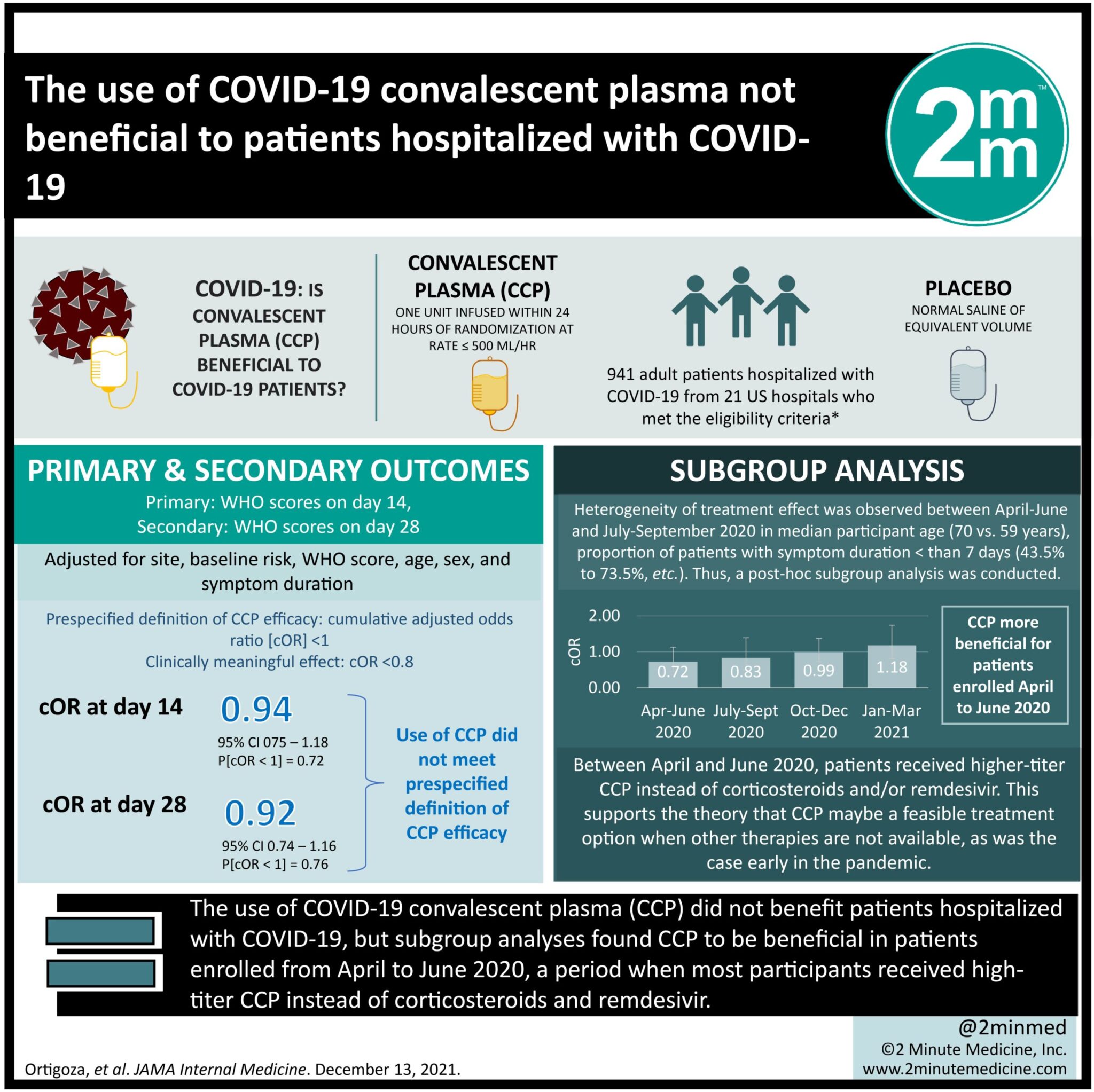
1. The use of COVID-19 convalescent plasma did not benefit patients hospitalized with COVID-19, assessed using the World Health Organization 11-point Ordinal Scale for Clinical Improvement.
2. However, the subgroup analyses found COVID-19 convalescent plasma to be beneficial in patients enrolled from April to June 2020, a period when most participants received high-titer COVID-19 convalescent plasma instead of corticosteroids and remdesivir.
Evidence Rating Level: 1 (Excellent)
Study Rundown: The use of convalescent plasma in prior pandemics as well as its biological plausibility has prompted the role for COVID-19 convalescent plasma (CCP) use in patients hospitalized with COVID-19. This randomized clinical trial assessed the safety and efficacy of CCP compared to placebo in hospitalized patients with COVID-19 receiving noninvasive supplemental oxygen. The primary endpoint was patient scores on the 11-point World Health Organization (WHO) Ordinal Scale for Clinical Improvement on day 14 where secondary endpoint was determined on day 28. An exploratory subgroup analyses was performed with respect to age, baseline WHO score, concomitant medications, symptom duration, CCP SARS-CoV-2 titer, baseline SARS-CoV-2 serostatus, and enrollment quarter. Among 941 hospitalized patients with COVID-19 requiring noninvasive oxygen supplementation, the use of CCP did not meet the prespecified definition of CCP efficacy (cumulative adjusted odds ratio [cOR] <1 and a clinically meaningful effect as cOR <0.8). However, the subgroup analyses found CCP to be beneficial in patients enrolled from April to June 2020, a period when most participants received high-titer CCP instead of corticosteroids and remdesivir. This supports the theory that convalescent plasma may be a more feasible treatment option during earlier stages of a pandemic or when other therapies are not yet in use or available. A limitation of this study was that the primary endpoint set at day 14 was likely too early for COVID-19, a virus known to have a more prolonged course and thus, highlighting the importance of the findings for the secondary endpoint at day 28.
Click to read the study in JAMA Internal Medicine
Relevant Reading: Convalescent plasma treatment of severe COVID-19: a propensity score–matched control study
In-Depth [randomized clinical trial]: This double-blind, placebo-controlled trial, CONTAIN COVID-19, included 941 (556 men [59.1%]; median age [IQR], 63 [52-73] years; 373 [39.6%] Hispanic, 132 [14.0%] non-Hispanic Black) hospitalized adults with COVID-19 from 21 US hospitals between April 2020 to March 2021. Eligible patients were those hospitalized for 3 or less days or presented 7 or less days after symptom onset and required noninvasive oxygen supplementation, where 473 individuals were randomized to placebo (normal saline) and 468 to CCP. For the primary endpoint, the cOR adjusted for site, baseline risk, WHO score, age, sex, and symptom duration was 0.94 (95% credible interval [CrI], 0.75-1.18) with posterior probability (P[cOR<1] = 72%). The cOR for the secondary adjusted outcome was 0.92 (95% CrI, 0.74-1.16; P[cOR<1] = 76%). Heterogeneity of treatment effect was observed through the exploratory subgroup analyses where on day 28, cORs were 0.72 (95%CrI, 0.46-1.13; P[cOR<1] = 93%) for participants enrolled from April to June 2020 and 0.65 (95%CrI, 0.41 to 1.02; P[cOR<1] = 97%) for those who did not receive remdesivir and corticosteroids. Adverse events were reported for 39 (8.2%) placebo patients and 44 (9.4%) patient receiving CCP (P = .57) and transfusion reactions occurred in 2 (0.4) placebo recipients and 8 (1.7) CCP recipients (P = .06).
©2021 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.