The iPrEx trial: Preexposure prophylaxis reduces HIV transmission in men and transgender women who have sex with men [Classics Series]
1. In human immunodeficiency virus (HIV)-seronegative men and transgender women who have sex with men, preexposure prophylaxis with antiretrovirals significantly reduced the risk of HIV infection when compared with placebo.
2. There were similar rates of serious adverse events in both groups, though patients taking antiretroviral preexposure prophylaxis were significantly more likely to experience nausea and unintentional weight loss.
Original Date of Publication: December 2010
Study Rundown: Despite advances in treatment and measures to reduce the risk of transmission, HIV continues to be spread at high rates with approximately 2.7 million new infections occurring annually worldwide. Postexposure prophylaxis is recommended after exposure to HIV-infected fluids, though this requires recognition of exposure and for therapy to start within 72 hours. Preexposure prophylaxis, that is taking antiretroviral medications to prevent infection, has been proposed as a means of further reducing HIV transmission. The Preexposure Prophylaxis Initiative (iPrEx) trial explored the effects of preexposure prophylaxis with a combination of emtricitabine (FTC) and tenofovir (TDF) in men and transgender women who have sex with men at high-risk of HIV infection as compared with placebo.
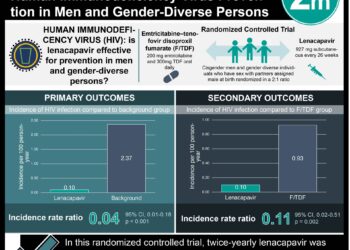
In summary, individuals who were randomized to receive FTC-TDF experienced significantly lower risk of new HIV infection than those were treated with placebo. Of the people randomized to the FTC-TDF group, it was found that those with detectable levels of the study drug had an even larger reduction in the risk of HIV infection when compared with those who did not have detectable levels of the study drug. This was the first randomized trial to explore the effects of preexposure prophylaxis with FTC-TDF. Since its publication, several other randomized trials have been published to support the utility of preexposure prophylaxis in other populations, including heterosexuals, serodiscordant couples, and injection drug users. Preexposure is now commonly recommended in individuals at high-risk of HIV infection, alongside other preventive methods.
Click to read the study in NEJM
In-Depth [randomized controlled trial]: A total of 2499 participants were enrolled from 11 sites in 6 countries (i.e., Peru, Ecuador, South Africa, Brazil, Thailand, United States). Individuals were included if they were male at birth and had sex with men, ≥18 years of age, HIV-seronegative, and at high-risk for acquisition of HIV (e.g., high number of sexual partners, unprotected receptive anal intercourse, transactional sex, known partner with HIV). At each follow-up visit, participants received HIV testing, testing and treatment for other sexually transmitted infections, and counseling regarding safe sexual practices. Participants were randomized to treatment with daily emtricitabine (FTC) and tenofovir (TDF) combination therapy or placebo.
Study participants were followed for 3324 person-years, with the median duration of observation being 1.2 years. HIV seroconversion was noted in 110 patents, though 10 had HIV RNA present in their baseline samples. Of the 100 individuals with emergent HIV infection, 36 were in the FTC-TDF group and 64 were in the placebo group, which represented a relative reduction in incidence of 44% (95%CI 15 to 63%, p = 0.005). In the FTC-TDF group, those with detectable levels of the study drug experienced a relative reduction in HIV risk of 92% (95%CI 40 to 99%, p < 0.001) when compared to those without detectable levels. There were no significant differences between the two groups in the rates of serious adverse events (p = 0.57). Those in the FTC-TDF group had significantly higher rates of nausea and unintentional weight loss (p = 0.04).
Image: PD
©2015 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.




![Paracetamol (Tylenol) ineffective for low-back pain [PACE Study]](https://www.2minutemedicine.com/wp-content/uploads/2014/07/479px-Extra_Strength_Tylenol_and_Tylenol_PM-75x75.jpg)