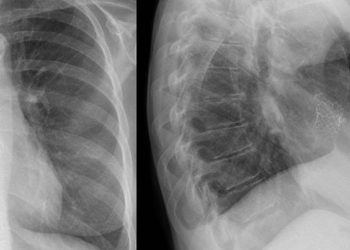
Tenecteplase is equivalent to placebo between 4.5 and 24 hours after stroke onset
1. In this randomized controlled trial, tenecteplase administered at 4.5 to 24 hours after stroke onset to patients with an occluded middle cerebral artery (MCA) or internal carotid artery (ICA) was not superior to placebo.
2. Tenecteplase did not differ from placebo with regards to rates of death and symptomatic intracranial hemorrhage.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Intravenous thrombolysis with tissue plasminogen activators, such as tenecteplase, is the standard of care for ischemic stroke within 4.5 hours after onset. Endovascular thrombectomy (EVT), conversely, is utilizable within 24 hours after stroke onset for large-vessel occlusions with evidence of salvageable tissue. It is unclear if tenecteplase, administered beyond 4.5 and up to 24 hours within this patient population, offers additional clinical benefits. This randomized controlled trial compared tenecteplase against a placebo administered at 4.5-24 hours to patients with an MCA or ICA ischemic stroke with imaging evidence of salvageable tissue being considered for EVT. By day 90, there was no significant difference in the disability assessment modified Rankin scale between the two trial groups. Additionally, the incidence of death and symptomatic intracranial hemorrhage was comparable between the groups, as was the rate of adverse events. These results represented a key departure from existing evidence demonstrating the benefit of EVT combined with tenecteplase administered within 4.5 hours from ischemic stroke onset. Overall, when administered outside this time window at up to 24 hours, tenecteplase did not offer additional clinical benefits compared to placebo.
Click here to read the study in NEJM
Relevant Reading: Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke
In-Depth [randomized controlled trial]: The current study was a randomized placebo-controlled trial investigating tenecteplase administered to patients with ischemic stroke 4.5 to 24 hours from stroke onset. Patients 18 years of age and older with independent function before the stroke who had an ischemic stroke attributable to occlusion of the MCA and/or ICA, with evidence of salvageable brain tissue demonstrated on perfusion imaging, and could be treated with tenecteplase 4.5 to 24 hours from when they were last known to be well were eligible for inclusion. Exclusion criteria included known bleeding diatheses, severe hypertension, EVT initiation before randomization, or hypersensitivity to tenecteplase. 458 patients, 77.3% of whom eventually underwent EVT, were randomized 1:1 to receive intravenous tenecteplase (0.25mg/kg body weight) or placebo as a bolus over five seconds. The primary outcome was the score on the modified Rankin scale at day 90. Overall, the median score on the modified Rankin scale was 3 in both groups at 90 days (common odds ratio, 1.13; 95% Confidence Interval [CI], 0.85 to 1.57; p=0.45). Functional independence was achieved in 46.0% of patients in the tenecteplase group and 42.4% in the placebo group (adjusted odds ratio [AOR], 1.18; 95% CI 0.80 to 1.74). The rate of recanalization at 24 hours was higher in the tenecteplase group (76.7%) than in the placebo group (63.9%) (AOR, 1.89; 95% CI, 1.21 to 2.95). Nonetheless, the two groups had similar rates of angiographic reperfusion after EVT (89.1% for tenecteplase and 85.4% for placebo). Regarding safety, the incidence of mortality did not differ significantly between tenecteplase (19.7%) and placebo (18.2%), nor did the incidence of symptomatic intracerebral hemorrhage (3.2% and 2.3%, respectively). These results showed that tenecteplase given to patients with large-vessel ischemic stroke between 4.5 and 24 hours from onset did not improve clinical outcomes compared to placebo.
Image: PD
©2024 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.