The majority of FDA-approved drug trials recruit from low- and middle-income countries
1. This study found that a high proportion of Food and Drug Administration (FDA) drug trials recruit participants from low- and middle-income countries (LMICs).
2. Cardiovascular trials had the highest proportion of participants recruited from LMICs.
Evidence Rating Level: 1 (Excellent)
Study Rundown: Nearly one-third of phase three trials sponsored by large pharmaceutical companies recruit participants from outside of the United States. Many drugs used in North America are also tested abroad, including (LMICs). While recruiting from LMICs can increase population diversity, expedite recruitment, and lower research costs, there are also ethical issues with recruiting from LMICs. These include exposure to unproven treatments and exploitation of these participants. However, there is a gap in knowledge as to understanding the extent to which pivotal trials, defined as trials that support a determination of efficacy, recruit from LMICs, and the proportion of participants from each country need to be better characterized and consistently reported. Overall, this study found that most pivotal trials for cancer, cardiovascular disease, and neurology recruited participants from LMICs. This study was limited by only studying FDA-approved drugs related to cancer, cardiovascular, or neurologic conditions and included only a sample of cancer drugs. Nevertheless, these study’s findings are significant, demonstrating that most pivotal trials for FDA-approved drugs recruit participants from LMICs.
Click to read the study in AIM
Relevant Reading: Participation of African American Persons in Clinical Trials Supporting U.S. Food and Drug Administration Approval of Cancer Drugs
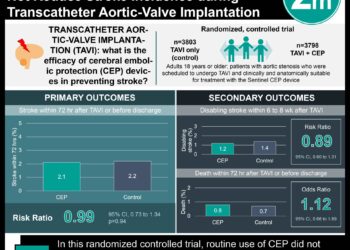
In-Depth [cross-sectional analysis]: This cross-sectional analysis utilized the FDA database “Compilation of CDER New Molecular Entity (NME) Drug and New Biologic Approvals” to identify all drugs and biologics initially approved from 2012 to 2019 for cancer, cardiovascular diseases, and neurologic conditions. Participant information was extracted from the publication reporting primary efficacy results (such a publication was found for every trial in the sample), medical and statistical reviews from FDA approval records, and ClinicalTrials.gov. The primary outcome measured was the proportion of pivotal trials enrolling participants in LMICs. Outcomes in the primary analysis were assessed by determining the proportion of trials recruited from at least one LMIC during their enrollment period. Based on the primary analysis, 56% in cancer, 79% in cardiovascular disease, and 56% in neurology recruited from an LMIC. For multi-country trials, country-level enrollment figures were unavailable for 71 trials (55%). For those reporting per country enrollment, the percentage of participants recruited from LMICs was 8% for cancer trials, 36% for cardiovascular trials, and 17% for neurology trials. Overall, shit study demonstrates that many pivotal trials for FDA-approved drugs recruit participants from LMICs, though more public reporting of country-level information on recruitment is needed.
Image: PD
©2022 2 Minute Medicine, Inc. All rights reserved. No works may be reproduced without expressed written consent from 2 Minute Medicine, Inc. Inquire about licensing here. No article should be construed as medical advice and is not intended as such by the authors or by 2 Minute Medicine, Inc.



![2MM: AI Roundup- AI Cancer Test, Smarter Hospitals, Faster Drug Discovery, and Mental Health Tech [May 2nd, 2025]](https://www.2minutemedicine.com/wp-content/uploads/2025/05/Untitled-design-350x250.png)